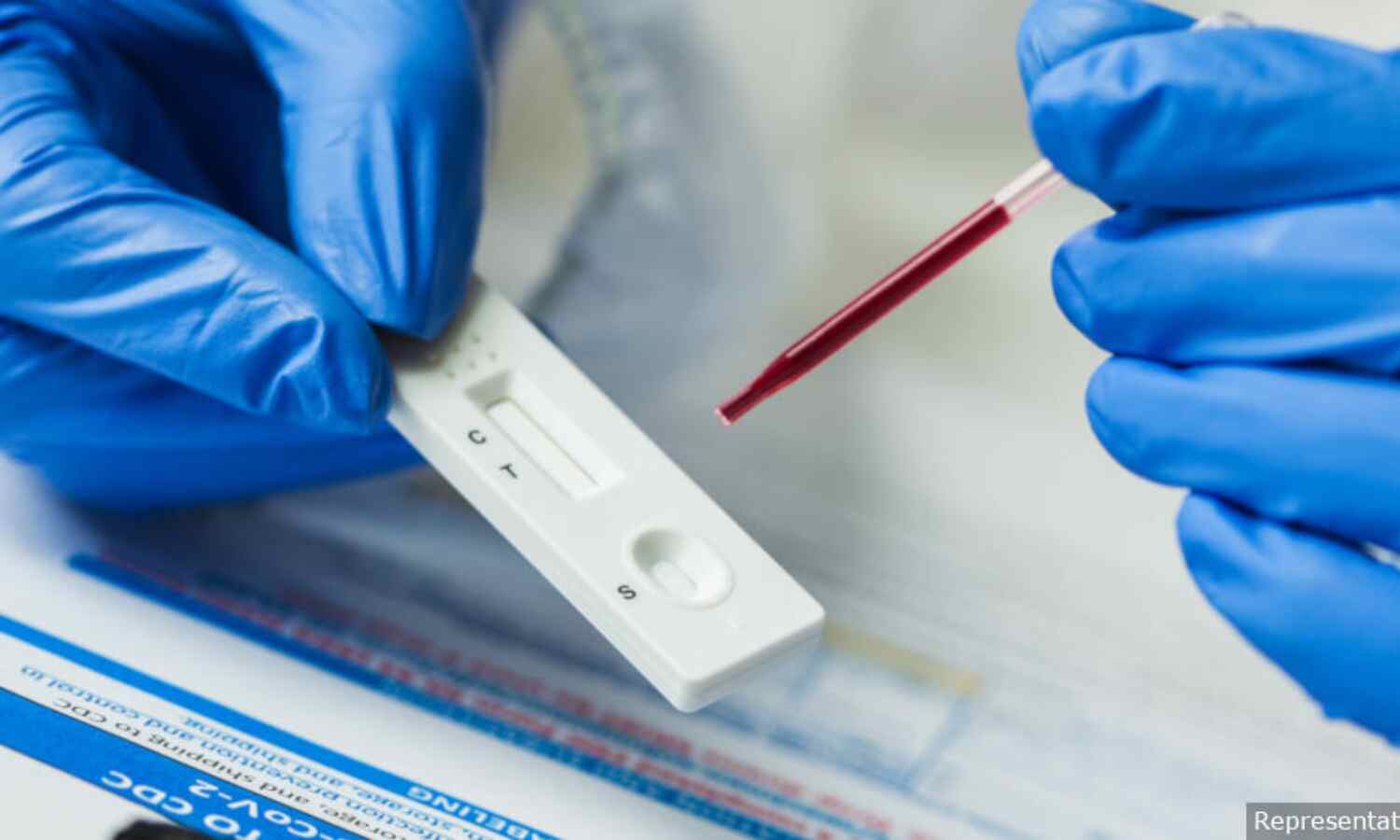
Doubts Over Antibody Testing Could Weaken India’s COVID-19 Strategy
New Delhi: States across India are either increasingly sceptical about antibody testing for COVID-19 or are reluctant to use it, IndiaSpend has found in conversations with several health officials. Antibody tests give vital information about a contagion’s spread, and this wariness could have significant implications for India’s current and future strategies to deal with COVID-19, said experts.
All through April 2020, the government had maintained during press conferences that a large tranche of kits was on its way from China. The delivery was delayed but on April 16, 2020, the antibody kits finally arrived in India. However by end-April, the enthusiasm for antibody testing waned after the government announced that the kits from two Chinese companies were faulty and asked states to stop using them. Now, states are reluctant to even put out tenders for antibody tests, we found.
“We have no plans of procuring any more rapid antibody tests right now,” said P. Umanath, head of the Tamil Nadu Medical Services Corporation. Tamil Nadu had placed an order for 50,000 rapid antibody tests from Wondfo, a Chinese company, but cancelled it when the Indian Council of Medical Research (ICMR) issued a circular blacklisting the company.
The Telangana government has not allowed antibody testing at all. “We have not done any so far and don't plan on it,” said Srinivas Rao, director of public health for the state. “Between RT-PCR testing for current positive cases and the lockdown, we think we can manage.” Telangana has extended the lockdown in the state until May 29, 2020. The fourth phase of the nationwide lockdown “will be completely redesigned, with new rules”, the Prime Minister said on May 12, 2020.
A state government official in Maharashtra handling the COVID-19 response--a medical doctor himself--told IndiaSpend off the record that antibody testing would not be much use since it has no role in diagnosis. Likewise, government officials from Delhi, Bihar and Karnataka who spoke to IndiaSpend were sceptical even about using the technology for surveillance.
Chhattisgarh was among the few states to show enthusiasm for the technology in the early days of the pandemic. “Surveillance by rapid testing kits helped us in identifying more positive cases amongst migrant workers in Surajpur,” tweeted state health minister T.S. Singh Deo. The state had procured 75,000 kits from a South Korean company, SD Biosensors, at Rs 337 plus GST each, without waiting for ICMR supplies. The ICMR later sent the state 4,800 of the Chinese kits; the state used 300 and returned the rest after the order for recall, said a source in the state health department who did not want to be named.
Now at 74,281 cases of COVID-19 in India and 2,415 deaths as of May 8, 2020, and into the third extension of the nationwide lockdown, the Indian government continues to validate antibody kits but has not been putting out tenders to buy any.
In non-critical situations, antibody testing performs an important function in epidemiology and public health. It provides answers to questions such as: Who has been exposed to the disease and in which areas? Which areas need to be further contained and which can be opened up? Who needs to be first in line to receive the vaccine if it is developed? The answer to these questions will decide how countries respond to the pandemic in the short and long run.
However, in the current COVID-19 crisis, the technology for antibody tests is still being developed and the results are not entirely robust, the World Health Organization (WHO) has said. But once the tests are validated, and information from them starts becoming more reliable, the results will become important indicators of how the campaign against COVID-19 should unfold, as we explain in detail later.
IndiaSpend has sent queries to the ICMR regarding its stand on antibody testing and will update this report when the institute replies.
Why antibody kits may fail
When the ICMR blacklisted two Chinese companies--Wondfo and Livzon--its press statement only said that the kits were “under-performing” with “wide variation in their sensitivity, despite early promise of good performance”.
The importer of these kits questions the ICMR’s decision. “The National Institute of Virology took about 100 samples from five batches to do their validation tests of these kits,” said Suresh K., the proprietor of Chennai-based Matrix Labs, which imported the kits from China. “They didn't find any problem and validated these kits. Now they are saying there was a fault in the kits but they won’t give us a detailed technical report outlining the faults. How do they expect us to believe this? They have also not paid for the 276,000 kits which were delivered to them.”
But there are several ways an antibody test can fail, and not just due to manufacturing issues. As the name suggests, these kits are supposed to give nearly instant results. They are used on the field, not in labs. And for many antibody tests, and especially for novel diseases such as COVID-19, this means a toss-up between speed and accuracy given that information about the SARS-CoV-2 virus is still emerging.
An antibody test can also “under perform” due to errors in how the kits were transported, stored or used on the field. For example, the blood for antibody tests needs to be drawn correctly on the field. “The prick must be made with some force so that blood is not diluted,” said Vineeta Bal, an immunologist at the Indian Institute of Science Education and Research. “A person in the early days of infection will not have as many antibodies so if the prick is not done with the right force, the tissue fluid will dilute the blood and an antibody test could then come as negative.”
Another way in which kits can give poor results is if batch consistency is not maintained and the ICMR has cautioned companies about this. Reagents, which are chemicals used in antibody testing, also need to be handled at the right temperature. Some antibody tests will have multiple reagents that need to be maintained at different temperatures.
On April 24, 2020, the WHO issued a complex scientific brief. It did not deny basic immunology that antibodies are produced when a person is exposed to SARS-CoV-2, but said the mere presence of antibodies does not imply immunity to the virus and that research on this is still under way.
The WHO also cautioned against the use of antibody tests in their current stage of development, saying that they “need further validation” to determine their accuracy and reliability. Given this lack of sufficient evidence, countries may not be able to use this information to give ‘immunity passports’ which could allow people to return to work once lockdowns are lifted, the WHO added.
Antibody testing still has critical uses
However, despite its reservations, the WHO went on to express support for countries conducting studies that test for SARS-CoV-2 antibodies at the population level or in specific groups. These studies “are critical for understanding the extent of--and risk factors associated with--infection”, the organisation said. “These studies will provide data on the percentage of people with detectable COVID-19 antibodies.”
RT-PCR tests and antibody tests are both essential but not interchangeable. RT-PCR testing, which the government calls the “gold standard” for diagnosis of COVID-19, is vital, but an April 11 government statement said it cannot always identify asymptomatic infections or infections in people who may have now recovered. Some 80% of COVID-19 cases in India have been asymptomatic, the ICMR has said.
This is where antibody tests can help because they look for antibodies even when an individual does not display the symptoms of the infection or has already recovered. Given that only those in a critical state are likely to be admitted to hospital for COVID-19, it is possible that many will recover on their own and at home. These people may never get diagnosed and be counted among India’s positive cases. But if antibody testing is done in the community, it will pick up these missed cases.
An efficacious antibody test for COVID-19, Bal said, can provide two valuable data points: Who has been exposed to the virus? Have they developed the COVID-19-specific immune response? These data are important to doctors, epidemiologists and policy makers, in both the short, medium and long term.
The ICMR announced on May 10 that it was going to do surveillance and antibody detection via a new ELISA test developed by the government. ELISA testing is another technology to check for the presence of antibodies. But it is not a ‘rapid’ test, it is done in the lab and not on the field. “The test has an advantage of having much higher sensitivity and specificity as compared to the several rapid test kits which have recently flooded the Indian market,” said the press release about the new test.
The government also announced that it would undertake district surveillance by ELISA tests for antibodies: “There is a need to establish systematic surveillance for SARS-CoV-2 infection in all districts of the country.”
Implications for policy decisions
Although the numbers of COVID-19 cases have been rising daily, the government has been asserting at press conferences that India’s curve on COVID-19 is “relatively flattening” and due to fall to zero by May 16, 2020.
Antibody testing presents India with a scientific approach on how to decide on its COVID-19 strategy but with the evidence around it still growing, and the recent bad experience with the technology, should policy makers embrace it with greater enthusiasm?
“It is important that we have this data on the prevalence of COVID-19 not just for posterity or as an academic exercise, but because we need to know right now: Where is the spread happening, do we need to immediately ramp up medical facilities in those areas, who are the vulnerable people?” said Ramana Dhara, professor at the Indian Institute of Public Health.
A well-working antibody test could also save money on testing, said Bal. Until earlier this month, the government’s containment plan required that a person who tests positive for COVID-19 once via the RT-PCR test can be discharged only if they test negative twice consecutively after that, also with RT-PCR (the policy has been revised this month to reduce testing before discharge). Labs can charge up to Rs 4,500 for each test. But if we had a reliable antibody test by now, the RT-PCR test could be followed by the faster and cheaper antibody test, to determine if the person is safe to discharge, said Bal.
In the medium term, antibody test results are also directly linked to decisions on the lockdown. If scientists conclude that the antibodies produced also offer immunity to COVID-19, India can confidently decide on lifting the lockdown. “COVID-19 is causing various problems, not just deaths immediately linked to the disease but also human costs due to the lockdown,” said Bal. “Industry has to re-start in India and focusing on antibody testing could help us figure out how soon to do that and in which areas.”
In the pandemic’s trajectory, results from antibody testing will tell the government whether the ‘curve’ of the pandemic is likely to keep rising, flattening or dropping.
In the long term, understanding the immunology around SARS-CoV-2 and developing better antibody tests will help us understand re-infections, subsequent waves of COVID-19 and the possibility of populations acquiring herd immunity (when a significant section of population has acquired immunity, they stop transmitting infection, thereby offering protection to even those not yet immune) and immunity passports (when those with immunity can be seen as safe to move around again in society).
Whenever a vaccine is ready, antibody testing will be key to determining who receives it. “If antibodies do provide protection against COVID-19, then vaccines can be given to those vulnerable populations who have not developed these antibodies,” said Bal. The government will need to do antibody testing to get the baseline area-wise data on positive cases, said Dhara. “This then tells us who needs a vaccine and if a vaccine has been effective in that area,” he added.
“It is in everyone’s interest--from industry to science to public health--to develop and use antibody tests,” Bal added.
(Bhuyan is a special correspondent at IndiaSpend.)
We welcome feedback. Please write to respond@indiaspend.org. We reserve the right to edit responses for language and grammar.