In 5 Years, Threat Of Drug-Resistant Superbugs Doubles
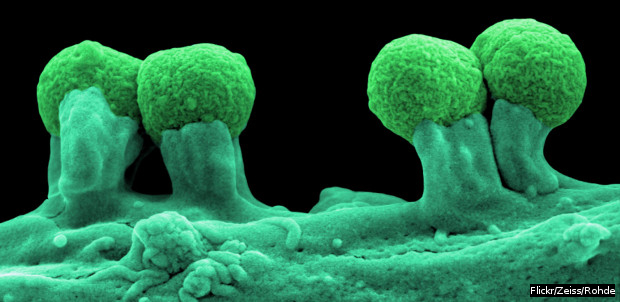
The pathogen Escherichia coli attached to the wall of a cell.
A 72-year-old woman in Bengaluru consulted a hospital physician about a severe skin infection and fever. She had previously consulted a couple of general practitioners, who prescribed a course of penicillin for three days and fluoroquinolones—both antibiotics—for two days.
There was no relief.
So, the consultant ordered a culture sensitivity test of pus from the skin lesions to identify what was causing her ailment and figure out what antibiotics it would respond to.
Here’s what the report said:
- Pathogen: Klebsiella pneumoniae
- Susceptible to: No antibiotic
- Resistant to: All antibiotics, including advanced drugs like fluoroquinolones, carbapenems and even the last resort combination usually reserved for severe cases of ICU infection, colistin-tigecycline.
With nothing to offer the patient, save a prescription for paracetamol to keep her fever in check, the doctor sent the patient home, and asked her to return after a week.
In such cases, sometimes, the body’s immunity kicks in and throws off the infection, the physician, Sheela Chakravarthy, consultant (internal medicine) at Fortis Hospital, Bengaluru, told IndiaSpend.
Sometimes, resistance to one or more drugs abates, allowing treatment to be resumed. Chances of that happening are greater at home, not in the hospital, which is a more infectious space where sepsis—a disproportionate and potentially life-threatening immune response by your body to an infection—could set in, she explained.
Most patients, however, succumb to the infection.
Chakravarthy faces situations where she has nothing to offer patients, not because they are suffering from terminal illnesses, such as some forms of cancer, but even when they present with what should be curable infections, “almost every day”, she said.
What Chakravarthy described is the consequence of rampant, inappropriate consumption of antibiotics, spurring the development of superbugs, as the recently released State of the World’s Antibiotics Report 2015 affirms.
India is fast becoming home to superbugs
Escherichia coli, Klebsiella pneumoniae and Staphylococcus aureus are three of the deadliest pathogens facing humanity, according to the World Health Organisation (WHO). And India is gradually but increasingly becoming home to multi-drug resistant strains of these pathogens, according to the State of the World’s Antibiotics Report 2015.
Escherichia coli is notorious for causing food poisoning and urinary tract infections.
In 2010, 5% of Escherichia coli samples in India were resistant to carbapenems, last-resort antibiotics for bacteria that are resistant to first-, second- and third-line drugs. By 2014, 12% of E. coli samples were similarly resistant.
Klebsiella pneumoniae causes pneumonia, septicaemia and infections in the urinary tract, lower biliary tract and at surgical wound sites, to name a few.
While 29% of Klebsiella pneumonia isolates were resistant to carbapenems in 2008, this increased to 57% in 2014.
For comparison, fewer than 10% of Klebsiella pneumoniae infections in Europe are carbapenem-resistant.
Staphylococcus aureus can cause skin and soft tissue infections, bloodstream infections, pneumonia and surgical site infections. A particularly nasty strain of, methicillin-resistant Staphylococcus aureus (MRSA), is common in India and increasingly hard to treat.
MRSA was responsible for 40% of post-surgical site infections, according to a 2013 study by the Jawaharlal Nehru Medical College and Hospital, Aligarh.
Between 2009 and 2014, the incidence of MRSA in India has risen from 29% to 47%.
People with MRSA are 64% more likely to die than people with a non-resistant form of the infection, according to the WHO.
How ignorance is spurring the development of superbugs
“My understanding of antibiotic is that it stops bacteria growing in body…I think amoxicillin is for throat infection.”
--An urban participant of a study of perceptions about antibiotic use and resistance among urban and rural doctors, pharmacists and public in Vellore.
Mox, short for amoxicillin, has become a household word across India.
A little knowledge, however, is a dangerous thing. It encourages self-medication, even when medicine is unnecessary, such as when people suffer viral infections—against which drugs are ineffective. Most viral fevers dissipate on their own after a few days with rest, hot fluids and a check on the fever.
Consuming too many antibiotics contributes to pathogen drug resistance.
“Resistance is an outcome of accumulated use,” said Ramanan Laxminarayan, vice president, Research and Policy, Public Health Foundation of India, and director and senior fellow, Centre for Disease Dynamics, Economics & Policy, US, and co-author of the State of the World’s Antibiotic Report 2015.
Indians often rely on corner pharmacists, whose knowledge of dosages may be limited.
Here’s what a rural pharmacist participant of the aforementioned Vellore study said: “Amoxicillin, 6 tablets is to be taken [for full course].”
Amoxicillin’s full course depends on the kind and severity of bacterial infection.
When an antibiotic of lower strength or fewer pills than needed is prescribed, the body cannot fully eradicate the pathogen. Sensing it has come under attack, the bacterium responds by evolving into more resilient, antibiotic-resistant strains.
But with a course of antibiotics, say generic Amoxicillin, costing about Rs 160, close to a day’s wage in many states, and a doctor’s consultation costing anywhere between Rs 100 and Rs 1,000, more than a day’s wage in most places, patients are bound to cut corners.
Another Vellore study participant summed up the situation thus: “If I have money I go to hospital. If not, I get medicine from pharmacy shop. If I get better, I stop and keep for future use.”
Stopping a course of drugs mid-way also contributes to antibiotic microbial resistance.
In a 2015 study in Chennai, 70% respondents confessed to stopping the medication when they felt better. Only 57% completed the antibiotic course.
“Less is more”: the key to preserving antibiotic efficiency
Educate health professionals, policy makers and the public on sustainable antibiotic use, says the State of the World’s Antibiotics Report 2015.
That is sensible advice.
Denmark and Sweden boast of low rates of antibiotic use and near-zero rates of antibiotic resistance because the risks of antibiotic overuse are widely known.
Instituting regulations on antibiotic use has reduced the proportion of MRSA in Europe and the US by about a fifth over the last eight years.
India requires more stringent regulations for antibiotic use.
It isn’t enough to tell physicians that they should prescribe antibiotics only when essential to cure bacterial infections. The right way is to order a culture sensitivity test, which costs money, and the patience to wait for the result.
“Patients want instant and cheap relief, and are willing to shop around for a doctor who obliges,” said Himanshu Shekhar, medical director, SCI International Hospital, New Delhi.
“Some judge doctors on how fast the prescribed medicine cures. Practice pressures lead many doctors to prescribe advanced drugs, without getting a culture-sensitivity test done.”
So, it’s also not enough to have 24 advanced antibiotics, including third- and fourth-generation cephalosporins, carbapenems, and newer fluoroquinolones, under the ambit of Schedule H1 of the Drugs & Cosmetic Rules, 1945, with effect from March 1, 2014.
That means these drugs cannot be sold over-the-counter, but they are still freely prescribed.
Chakravarthy’s suggestion: “Make Schedule H antibiotics available only through hospitals and health centres.”
“Changing antibiotic usage behaviours is critical to preserve the efficacy of existing and new drugs,” proposed Laxminarayan.
India also sorely needs regulations to check antibiotic use in animals raised for human consumption, to meet the State of the World’s Antibiotic Report 2015 recommendation to reduce and eventually phase out sub-therapeutic antibiotic use in agriculture.
Sub-therapeutic use implies mixing antibiotics in animal feed to make them grow faster and to prevent infections from devastating the herd or flock.
India is among the world’s five biggest consumers of antibiotics for livestock. IndiaSpend has earlier reported increasing evidence of antibiotic-resistant bacteria in animals in India, and how this impacts humans.
“Using antibiotics to make animals fatter faster is a waste of a precious resource,” said Laxminarayan.
How surgeons contribute to antibiotic resistance
Surgical antibiotic prophylaxis refers to the prescribing of antibiotics before, during and after operations to prevent infection.
Between 19% and 86% of patients in hospitals in India receive “inappropriate antibiotic prophylaxis”, according to the State of the World’s Antibiotics Report 2015. A prophylactic is preventive treatment for a disease.
Ideally, antibiotic prophylaxis should be administered as a single dose within 60 minutes of the skin incision. However, a 2013 Mangalore-based study found timing adhered to in 22% of cases in a government hospital, 64.9% cases in a medical-college teaching hospital and 80.7% of patients in a tertiary care corporate hospital.
“Smart antibiotic prophylaxis also includes choosing narrow-spectrum antibiotics to target the organism most likely to present concerns based on the kind of surgery being performed, this avoids needless exposure to antibiotics for the other microbes and helps prevent resistance,” said Vimesh Mistry, assistant professor, Pharmacology, Baroda Medical College.
Staphylococcus aureus, which lives on the skin, is most likely to cause infection during surgery. But surgeons frequently make poor antibiotic choices.
“We found appropriateness of choice of antibiotic in 68% cases and 52% compliance with the in-house prophylaxis guidelines,” said Tanu Singhal, infectious diseases specialist, Mumbai, and co-author of another study on antibiotic prophylaxis conducted in PD Hinduja Hospital, Mumbai.
Other prophylaxis inaccuracies include the unnecessary prescribing of antibiotics, inaccurate dose and inaccurate duration of prescription.
“We logged 63% accuracy in prescription duration. Surgeons tend to prescribe antibiotics for too long fearing post-surgery infection,” said Singhal.
In the trade off between protecting the patient better and increasing the risk to society of a pathogen developing resistance, surgeons are choosing the former.
Needed: A back-to-the-basics approach to health
Reducing the need for antibiotics through improved water, sanitation and immunisation is another strategy recommended in the State of the World’s Antibiotics Report 2015.
“Vaccination against pathogens such as the diarrhoea-causing rotavirus and pneumonia-causing Klebsiella pneumoniae helps curtail antibiotic demand, thereby reducing the chances of resistant strains developing,” said Laxminarayan.
In Canada, the widespread use of pneumococcal conjugate vaccines for pneumonia in children has reduced the incidence of pneumonia caused by strains the vaccine covers.
However, just as antibiotic usage spurs the development of superbugs, vaccination is a double-edged sword.
Canada is seeing a rapid increase in the incidence of other strains of pneumonia not protected against by the vaccine.
So, it is better to focus on the basic constituents of health.
Making available clean drinking water and improving sanitation would prevent people from getting sick in the first place. India still has a lot to do on both these fronts.
Improving individual immunity is the best bet to ward off infections, and that is also achievable by healthier eating, exercising, healthier living and the better management of chronic conditions like diabetes and asthma that increase vulnerability to infections when they are not kept in check.
(Bahri is a freelance writer and editor based in Mount Abu, Rajasthan)
“Liked this story? IndiaSpend.org is a non-profit, and we depend on readers like you to drive our public-interest journalism efforts. Donate Rs 500; Rs 1,000, Rs 2,000.”